
TA2 pure titanium has good corrosion resistance, high specific strength, and strong fracture toughness. In large-scale industry, ship production and other fields have been widely used, because the metal in the processing process of a certain case, its structure mainly depends on the highest temperature of heating and different cooling speed of the organization evolution.

1. Experiment
1.1 Experimental materials
The experimental TA2 raw material is annealed (M), and the titanium plate with a thickness of 50mm is rolled. Its β transition temperature is about 920℃, and its microstructure is isoaxial α phase, as shown in Figure 1. The Vickers hardness of 5 random points in its cross section is shown in Table 1.
1.2 Experimental methods
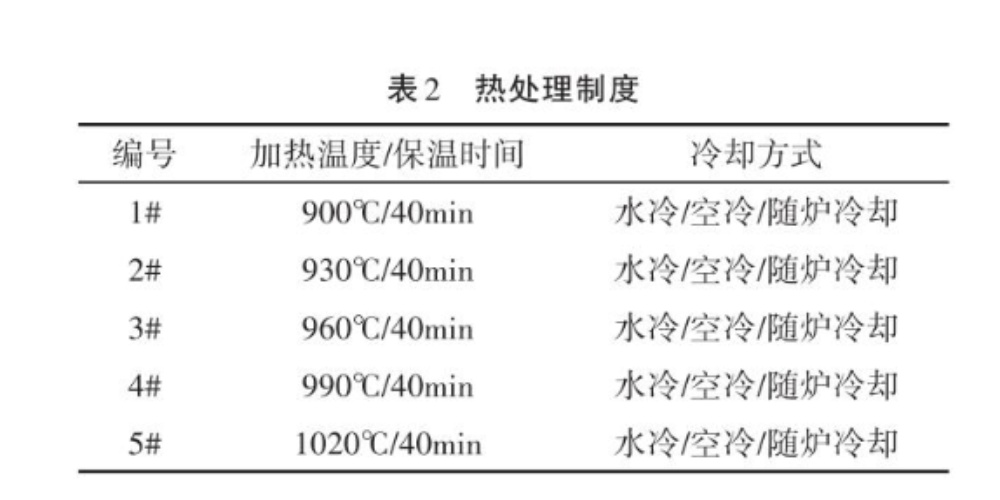
Cut the raw materials into 15x15mm small squares, a total of 15 pieces, divided into 5 groups, numbered 1#~5#, each group of 3 samples. The furnace temperature was respectively raised to the specified temperature point, a total of 5 temperature points, 900℃, 930℃, 960℃, 990℃, 1020℃, and each group of samples was put into it to hold heat for 40 minutes, and then a group of 3 samples were cooled to room temperature by means of water cooling (WO), air cooling (AC), and cooling with the furnace (FC), respectively. The heat treatment system is shown in Table 2.
The samples after heat treatment were tested for Vickers hardness (HV5), microstructure and original B grain size. Each sample was randomly marked with 5 hardness values, and then its average value was calculated. The microstructure was tested and photographed with a metallographic microscope.
2. Experimental results
2.1 Hardness
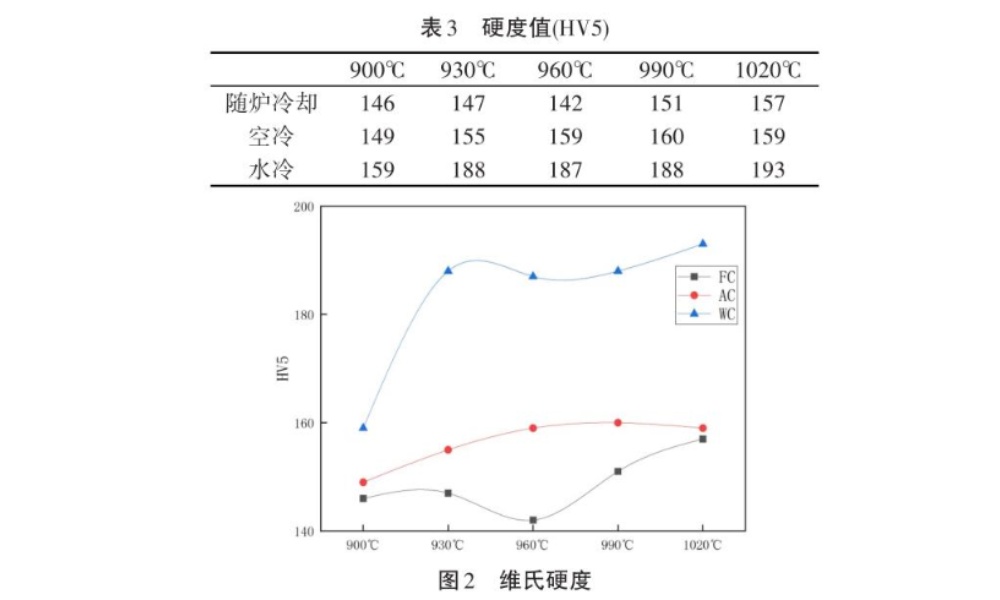
Under different temperatures and heat treatment systems, Vickers hardness values are shown in Table 3 and Figure 2.
2.2 Microstructure
At 900℃(below β transition temperature), the isoaxial structure is formed by furnace cooling, the isoaxial structure is formed by air cooling and part of the sheet a structure, and the water-cooled structure is composed of the isoaxial primary α phase and the fine sheet x phase.
At the temperature of 930℃~1020℃(above β transition temperature), the microstructure is composed of the whole laminae coarse α phase with the cooling of the furnace, and the microstructure has no obvious change with the increase of temperature. The air-cooled microstructure is also composed of the whole lamellar coarse α phase, and the microstructure has no obvious change with the increase of temperature, but the lamellar spacing of air-cooled microstructure is smaller than that with the furnace cooling at the same temperature. The water-cooled structure is composed of lamellar, lenticular and equiaxed α phases.
The microstructure under each system is shown in Figure 3. Figure 3(a)~3() shows the microstructure of furnace cooling, air cooling and water cooling at 900℃, respectively. The microstructure of 3(d)~3(f) is 930℃ with furnace cooling, air cooling and water cooling, respectively. Picture number and so on.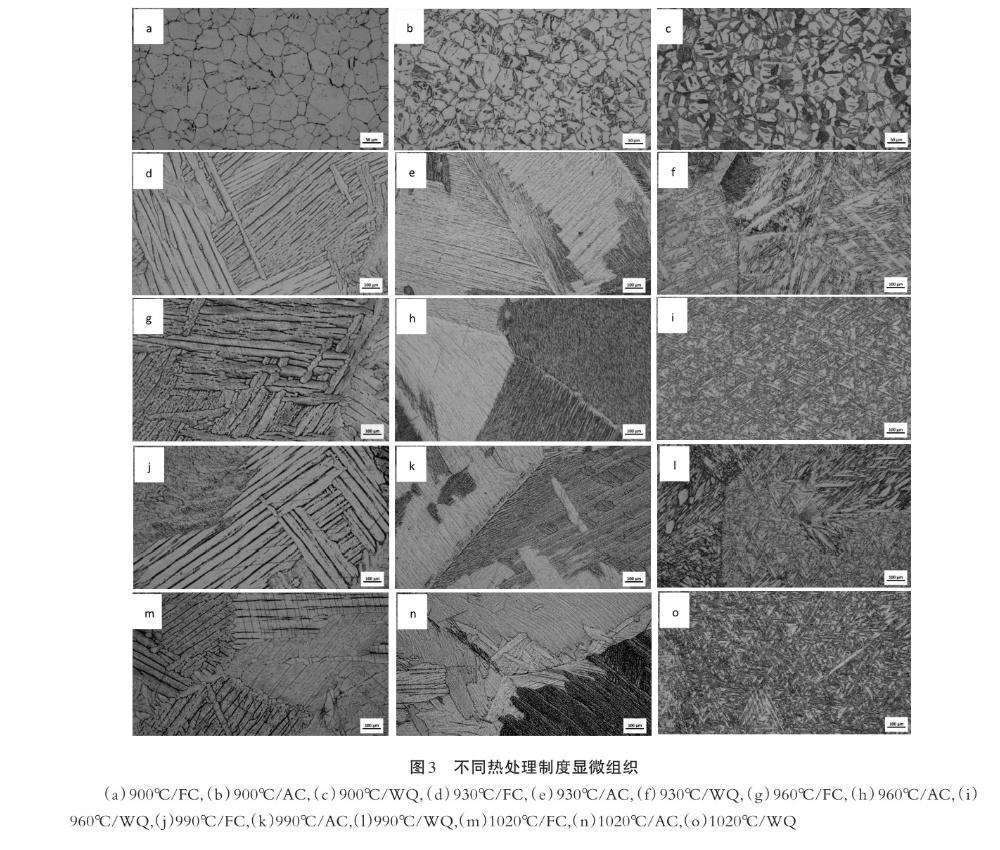
2.3 Primitive B grain
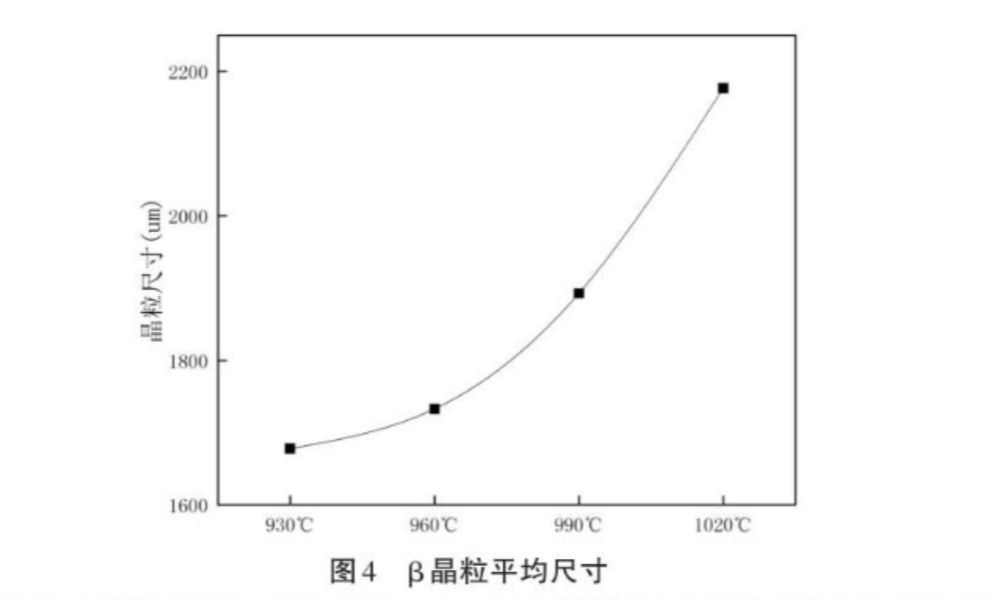
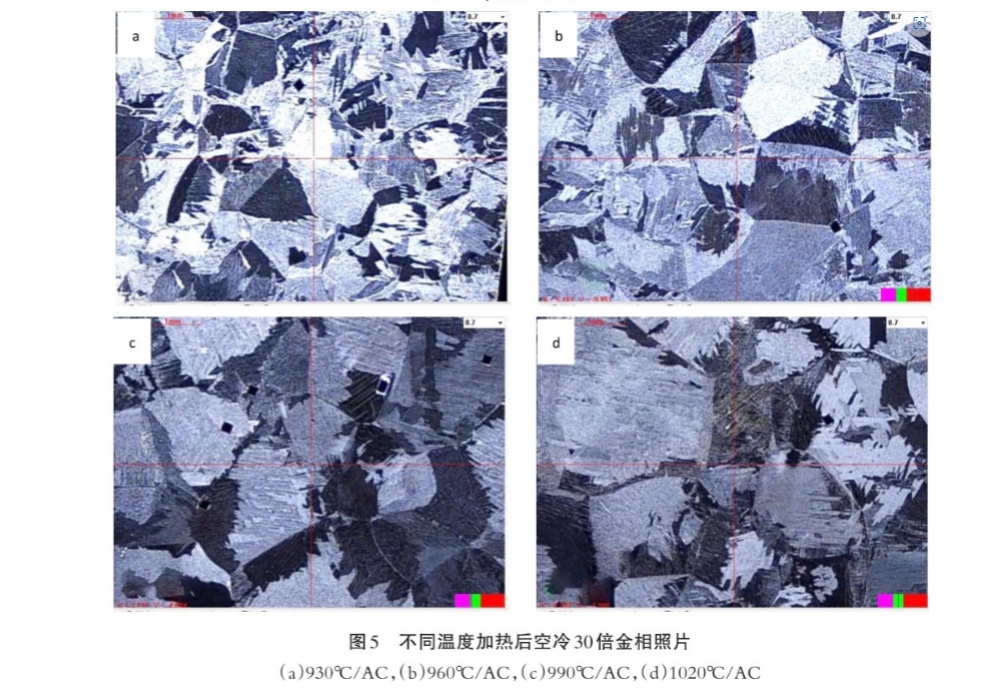
Under air cooling conditions of 930℃~1020℃, the original B grain outlined by the grain boundary α phase can be clearly seen, and the original β grain size at various temperatures can be measured, as shown in Figure 4. The metallographic photos are shown in Figure 5, and Figure 5(a)~5(d) are 930℃~1020℃ successively.
3. Conclusion and analysis
(1) At the same heating temperature, the Vickers hardness of TA2 pure titanium increases successively with furnace cooling, air cooling and water cooling. It can be seen from the microstructure that under the same temperature, equiaaxial crystals are formed with furnace cooling below the phase transition temperature, and thick lamellar structures are formed above the phase transition point, which will reduce the hardness of the material. The lamellar structures formed by air cooling are less than the lamellar spacing. It can increase the hardness, so the hardness will be greater than that of cooling with the furnace [3]; Water cooling forms lenticular and extremely fine lamellar structure, so the dislocation movement is greatly improved, thereby increasing the hardness, and its hardness value is significantly higher than that of cooling with the furnace and air cooling.

(2) Above the phase transition temperature, as the temperature increases, the hardness values are similar under the same cooling method. It can be seen from the microstructure that the α spacing of the lamellar layers is almost the same, and the overall microstructure is not different [4]. This is because the formation of the lamellar structure in the crystal is only related to the cooling rate, and has little relationship with the heating temperature.

(3) Above 930℃, with the increase of heating temperature, the original β grain will also increase, and the higher the temperature, the larger the grain trend, showing an accelerated growth trend. Because the higher the heating temperature, the higher the energy of grain boundary atoms, the greater the kinetic energy of boundary migration. The more obvious the trend of grain growth。
